Process Development and the Valita Titer Assay
Process development is a systematic approach to designing, optimizing, and scaling up a manufacturing process for the production of a specific product. It involves a series of activities aimed at understanding and improving the process parameters, conditions, and controls to achieve desired product quality, yield, and efficiency.
In the context of biopharmaceutical manufacturing, process development typically refers to the development of a bioprocess for the production of therapeutic proteins, antibodies, or other biologics. This includes cultivation of cells (such as mammalian cells, yeast, or bacteria), optimization of culture conditions, purification of the target protein, and formulation and final product processing.
In process development workflows the accurate, rapid, and high-throughput measurement of immunoglobulins, particularly immunoglobulin G (IgG), is essential to screen for productivity and to ensure the development of optimized and high-value processes.
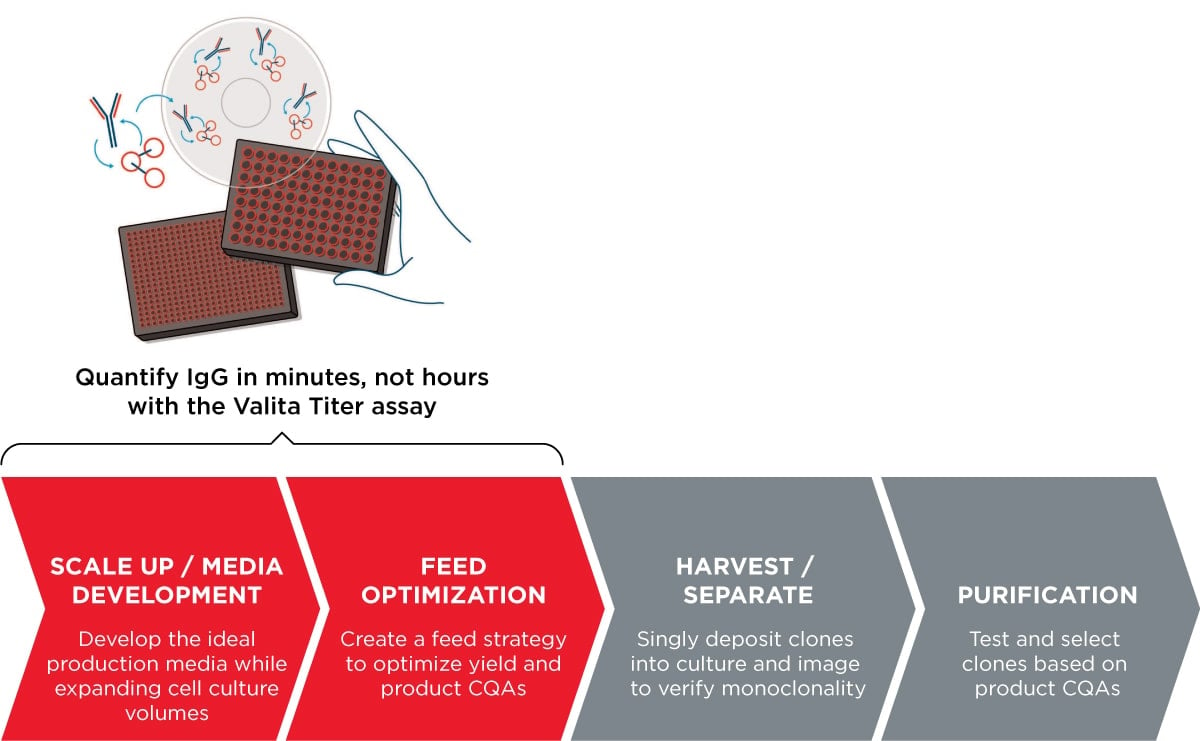
Enhancing Your Workflow
The Valita Titer assay enhances the antibody process development workflow by enabling rapid, high-throughput IgG quantification directly from crude cell culture sample. It supports the early-stage evaluation, optimization of culture conditions, scale-up/down assessment, process robustness evaluation, and troubleshooting.
- Early-stage process evaluation – By providing rapid and accurate measurements of antibody concentration, it enables you to quickly assess the performance of different conditions and select the most promising candidates for further optimization.
- Optimization of culture conditions – By measuring antibody concentrations in real-time, it provides insights into the impact of these conditions on antibody production, enabling you to identify the most favorable conditions for optimal productivity.
- Process scale-up and scale-down – By measuring antibody concentrations at different scales, it provides valuable information on the scalability of the process and helps in identifying potential issues or limitations that may arise during scale-up or scale-down.
- Process robustness evaluation– It can be used to assess bioprocess robustness by measuring antibody concentrations under different conditions, such as changes in temperature, pH, or dissolved oxygen levels.
- Process troubleshooting – By comparing antibody concentrations in different samples or process conditions, it helps identify the steps or parameters that may be responsible for the problem, enabling you to take corrective actions.
Insightful Resources
Rapid Rabbit IgG Quantification using the Valita Titer Assay
Valita Titer Assay Brochure
Increased throughput for IgG quantification using Valita Titer 384-well plates
Automating the Valita Titer IgG Quantification Assay on a Biomek i-Series Liquid Handling System
Learn More About the Valita Titer Assay

Introducing Valita Titer Assays
Valita Titer plate-based, 96- and 384-well assays that offer a rapid, cost-effective way to measure IgG

Automate With Ease
Easily integrate Valita Titer into your automated liquid handler and microplate reader
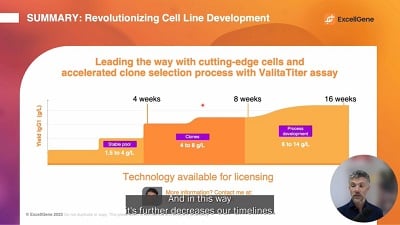
Cell Line Development Webinar
Speed up your clone selection process with the Valita Titer assay
Say Goodbye to Sample Preparation
Measure IgG titer straight from cell culture samples—no sample prep, additional reagents or wash required

Future of Bioprocessing
Let's dive into the world of monoclonal antibodies and explore the solutions that pave the way for innovative and effective therapeutics.
Let’s Accelerate Your Antibody Discovery and Development
Interested in a demo? Just want to ask a few questions?
We’re here to help, please reach out anytime.





